Dry and Wet Age-Related Macular Degeneration (AMD)
Wet AMD is preceded by dry AMD, a condition characterized by the development of yellow-white deposits under the retina, known as drusen, along with variable thinning and dysfunction of the retinal tissue, although lacking any abnormal new blood vessel growth.
In a subset of patients, dry AMD converts to wet AMD when new and abnormal blood vessels invade the retina. The abnormal new blood vessels originate beneath the retina, in a layer called the choroid, and infiltrate the overlying retinal layers. This abnormal new blood vessel growth is called choroidal neovascularization (CNV).

Progression from Dry AMD to Wet AMD
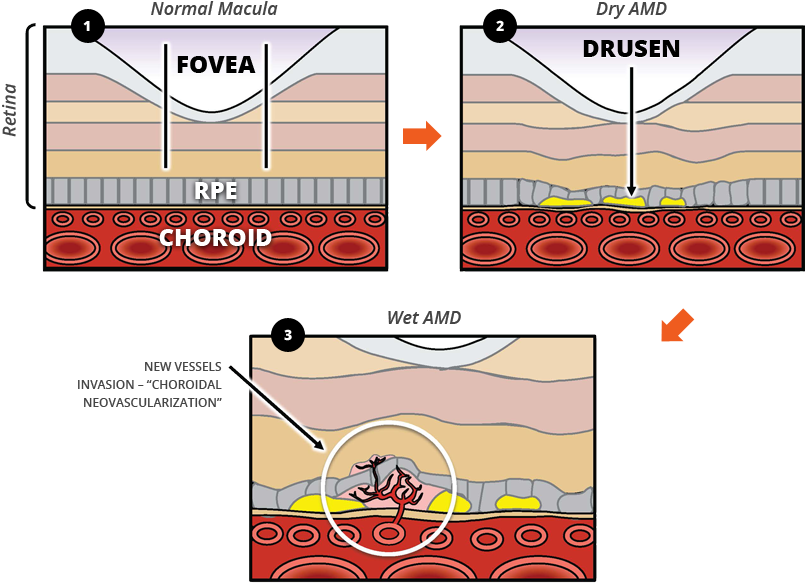
- In the normal macula, the thickness of the fovea (the central portion of the macula) and the retinal pigment epithelium (RPE, the layer between the fovea and choroid) are regular.
- Dry AMD occurs when yellow-white deposits called drusen form under the retina, thinning the RPE and making the thickness of the fovea irregular.
- Dry AMD converts to wet AMD when new and abnormal blood vessels emerge from the choroid and invade the retina – a process known as choroidal neovascularization (CNV).

Vision Loss in Wet AMD
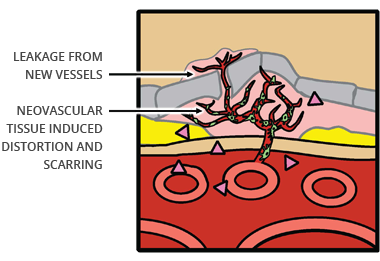
Abnormal new blood vessels that emerge during the process of choroidal neovascularization (CNV) often bleed and leak fluid into the macula, causing retinal distortion, scarring, destruction of the macula and loss of vision in patients with wet AMD.
Limitations of Available Therapies for Wet AMD
The use of anti-VEGF drugs, the current standard of care for wet AMD, has improved visual outcomes for patients who have been treated with these drugs as compared to untreated patients. Nevertheless, there remains a need for the improved management of wet AMD.
In clinical studies of the current standard of care, anti-VEGF monotherapy, in newly diagnosed wet AMD patients after one year of treatment:
- The majority do not achieve significant visual gain (≥ 3-Line Gain)
- Approximately 18 – 22% lose vision
- The majority do not achieve final visual acuity of 20/40 or better, which is necessary to obtain a driver’s license in many states
According to multiple studies published in the peer-reviewed journal Ophthalmology in 2013:
- 46% of patients with wet AMD lost additional vision approximately two years after the conclusion of a two-year Phase 3 anti-VEGF monotherapy clinical study
- 37% of patients with wet AMD had final visual acuity at the level of legal blindness (20/200 or worse) approximately five years after the conclusion of a two-year Phase 3 anti-VEGF monotherapy study
Resistance to Anti-VEGF Therapy
Limitation and/or resistance to anti-VEGF monotherapy, which could limit the clinical benefits of this therapy, may result from multiple mechanisms, including:
- Cell-based protection of neovascular endothelium from anti-VEGF effects
- Contribution of multiple non-VEGF mediators of neovascular angiogenesis
- Inflammation
Pre-clinical angiogenesis research suggests that pericytes covering the endothelium lining the neovascular complex locally produce VEGF and other factors involved in endothelial cell survival. As a result, pericyte coverage of the endothelium in angiogenic vessels could protect the neovascular tissue from the effects of anti-VEGF drugs. Therefore, stripping of pericytes may subject the unprotected endothelial cells to cell death from anti-VEGF therapy.
Research has also shown that PDGF plays a critical role in the recruitment of pericytes to newly formed vessels, covering the vascular endothelium. PDGF binds to a receptor on pericytes providing an important cell survival signal to the cells. Pericytes locally supply the endothelial cells with growth and survival factors, including VEGF, and play a major role in endothelial cell survival, support, and structure.
Therefore, pericyte stripping via PDGF inhibition may allow anti-VEGF therapy to be more effective, and lead to the regression and/or disruption of the abnormal blood vessels and enhance visual outcomes.
Other possible sources of anti-VEGF resistance include inflammation and increased levels of other growth factors and proteins not targeted by anti-VEGF drugs but involved in the complex orchestration of neovascular proliferation.
We believe Ophthotech’s lead clinical candidate, Fovista®, has demonstrated the ability to strip pericytes in pre-clinical models, and the potential to improve visual outcome compared to anti-VEGF monotherapy in non-pivotal clinical studies to date.