Fovista® Anti-PDGF Therapy Clinical Development
Ophthotech has completed a multicenter, randomized, double-masked, controlled Phase 2b clinical trial evaluating the efficacy and safety of Fovista® administered in combination with an anti-VEGF agent for the treatment of patients newly diagnosed with wet AMD. The trial included 449 patients at approximately 69 centers in North America, South America, Europe and Israel.
In this study, Fovista® 1.5mg administered in combination with Lucentis® (ranibizumab injection) demonstrated statistically significant superiority compared to Lucentis® monotherapy based on the primary endpoint of mean change in visual acuity from baseline at 24 weeks. Patients receiving the combination of 1.5 mg of Fovista® and Lucentis® gained a mean of 10.6 letters from baseline on a standardized chart of vision testing compared to a mean gain of 6.5 letters from baseline for patients receiving Lucentis® monotherapy, representing a 62% comparative benefit from baseline. Fovista® exhibited a favorable safety profile and no significant safety imbalances were observed for Fovista® combination therapy as compared to Lucentis® monotherapy.
Based on these results, Ophthotech has initiated a pivotal Phase 3 clinical program to evaluate the safety and efficacy of Fovista® 1.5mg in combination with anti-VEGF drugs compared to anti-VEGF monotherapy for the treatment of newly diagnosed patients with wet AMD.
Ophthotech has entered into a licensing and commercialization agreement with Novartis, granting the company exclusive rights to commercialize Fovista® in markets outside the United States. Ophthotech retains sole rights to commercialize Fovista® in the U.S. and will continue to lead the global Phase 3 clinical program and the potential U.S. registration of Fovista®. Ophthotech and Novartis will collaborate to seek regulatory approvals for Fovista® outside the U.S. For more information on the deal click here.
Fovista® fact sheet

How Fovista® Works
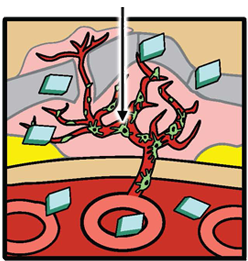
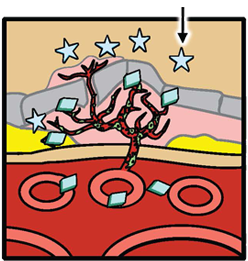
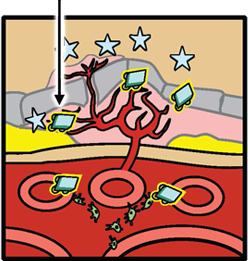
Fovista® is designed to target platelet derived growth factor (PDGF) and in combination with anti-VEGF drugs disrupt the formation of abnormal new blood vessels in wet AMD. It prevents PDGF from binding to its natural receptor on pericytes, thus causing pericytes to be stripped from newly formed abnormal blood vessels. Left unprotected, the endothelial cells are highly vulnerable to the effects of anti-VEGF drugs. Because of the ability of Fovista® to induce pericyte stripping from newly formed blood vessels, the administration of Fovista® in combination with anti-VEGF drugs is likely to inhibit abnormal new blood vessel growth associated with wet AMD more effectively than anti-VEGF drugs alone and may also enhance neovascular regression.
Abnormal new blood vessel formation and growth (neovascularization) is a hallmark of wet AMD. Pharmacology studies indicate that Fovista® binds to PDGF-BB, blocking the interaction of this growth factor with the pericyte cell surface receptor PDGF-ß. This results in stripping or death of the pericytes by interrupting the cell survival signals. In preclinical studies involving models of ocular neovascularization, concurrent inhibition of PDGF-BB and vascular endothelial growth factor A (VEGF-A) signaling was superior to inhibition of the VEGF-A pathway alone, and demonstrated the potential to induce neovascular regression.
PericyteAnti-VEGF Pericyte coverage protects new vessels from anti-VEGF-induced disruption PDGFFovista Fovista® is designed to inhibit PDGF Fovista® Bound to PDGF Fovista® inhibits PDGF, leading to pericyte stripping Fovista® + Anti-VEGF Combination Therapy Combination therapy with Fovista® and an anti-VEGF agent results in enhanced anti-angiogenesis and new vessel disruption and regression.


Rationale for Combination Therapy for Wet AMD
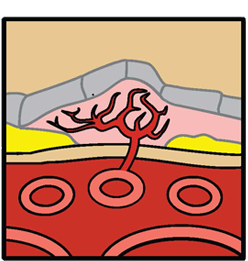
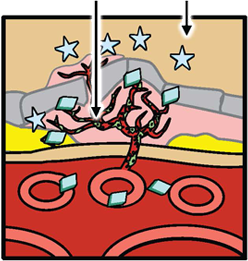
The abnormal new blood vessels that grow as part of choroidal neovascularization in wet AMD are predominantly made up of two cell types, endothelial cells and pericytes. The endothelial cells line the inside of abnormal new blood vessels; pericytes cover the outside.
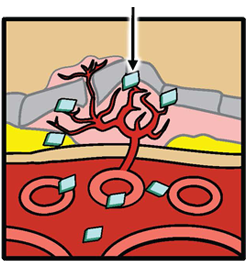
The steps to formation of new blood vessels are illustrated in the accompanying diagrams, which show cross-sections of the back of an eye and the chemical and cellular processes associated with the progression to wet AMD. First, vascular endothelial growth factor (VEGF) binds to a receptor on endothelial cells, causing them to proliferate and form new blood vessels. VEGF provides survival signals to endothelial cells and induces blood vessel permeability, causing the new blood vessels to leak.
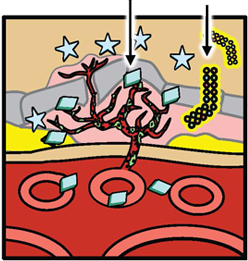
Next, platelet-derived growth factor (PDGF) binds to a receptor on pericytes, providing important cell survival signals while supporting and stabilizing the blood vessels. PDGF also recruits pericytes to the vessels, where they cover the endothelial cells and supply them with growth and survival factors (including VEGF), protecting the cells from any disruption that may be caused by anti-VEGF monotherapy.
VEGF induces new blood vessel growth and leakage PDGF PDGF levels rise Pericyte PDGF recruits pericytes Anti-VEGF Pericyte coverage protects new vessels from anti-VEGF-induced disruption.